World Stem Cell Institute worldstemcellinstitute.org in conjunction with World Stem Cell Clinic (India) worldstemcellclinic.com are conducting clinic trials on Fuchs disease “Comparison of endothelial cell layer pre and post Bone Marrow (BM) introduction to cornea” and “pre and post Platelet Rich Plasma/Growth Factors (PRP)”. The goal of this clinical trial is to compare the quality and number of endothelial cells during pre and post treatment to determine the efficiency of the treatment. In Vivo and Clinical Comparison of the quality and number of endothelial cells pre and post treatment to determine the efficiency of the treatment in Patients With Degeneration of the Corneal Endothelium.
Fuch’s Dystrophy
Fuch's Disease
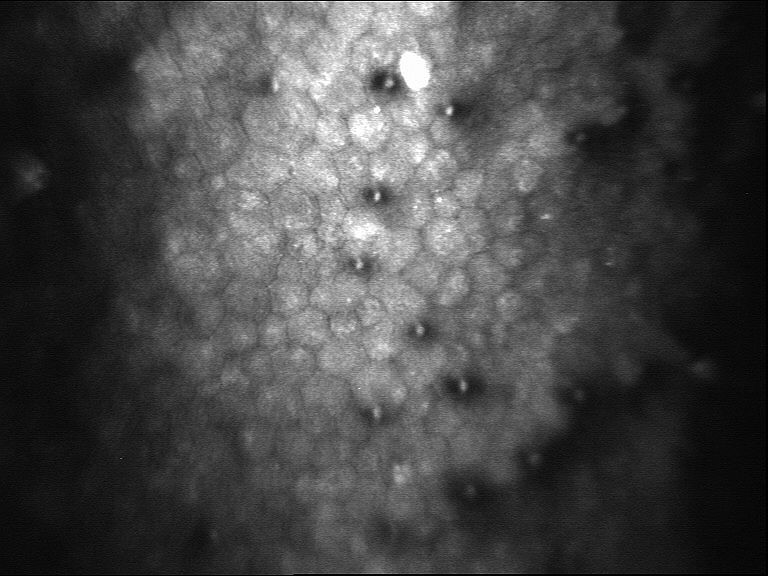
Testing Procedure
Primary Outcome Measures:
• The primary outcome is to determine the endothelial cell density of the Corneal Endothelium [ Time Frame: The measurement will be performed on the 6th day after the of start of treatment and then at the 20th days and then 30 days thereafter.]
Secondary Outcome Measures:
• Secondary study outcomes are immune rejection, as well as the incidence of corneal edema, corneal opacities and corneal neovascularisation. [ Time Frame: The measurement will be performed on the 6th day after the of start of treatment and then at the 20th days and then 30 days thereafter.]
·Visual acuity – Patient will be given an eye chart test to determine if their vision has worsened or improved and recorded at the Pre and Post exams.
·Glare test – Similar to the vision acuity test, a bright light is directed at patients eye while they read the characters on the chart. This test will determine the decrease or increase in vision related to glare and recorded.
·Grade or Guttata stage – An optical microscope called a slit lamp is used to examines the endothelial cells in the cornea. If there are irregularities, called guttae, on the back surface of the cornea are present the patients Fuchs’ dystrophy is assigned a “grade” or “stage” of zero through five. This number indicates the severity of dystrophy. A zero means there’s no disease while a five means the patients cornea is badly affected and this data will be recorded.
·Corneal cell count – A Specular Microscope and slit lamp will record and document the size and shape of the patient’s endothelial cells and measures the number of endothelial cells within a specific part of your cornea. All data will be photographed and recorded at the Pre and Post exams.

Criteria to be part of the study:
Inclusion Criteria:
• Male and Female patients between 50 and 85 years of age
• Clinically documented Fuch’s corneal dystrophy (what level 1-4?)
• Patient informed consent
• No contraindications *
Exclusion Criteria:
• Previous penetrating keratoplasty
• Corneal neovascularisation
• Pathologic changes in the anterior segment of the eye (anterior / posterior synechiae, uveitis)
• Glaucoma
• Aphakia
• Infectious diseases of the cornea
• Neurodermitis
• Participation of the patient in another clinical trial within the last four weeks that precede the recruitment
• The patient is unlikely to comply with the requirements of the protocol
• Previous or current abuse of medications, narcotics or alcohol
• Pregnancy
• Medications listed below
• Cancer either current or within the last 5 years
• Diabetes, unless well controlled
• Cataracts
• Kidney Disease
• Macular Degeneration
• Intraocular trauma including PHx
*Medications:
•Analgesics (eg, acetaminophen)
•Antibiotics (eg, cephalosporins)
•Diuretics
•Digoxin
•Disulfiram
•Heavy metals (eg, gold)
•Heparin
•Hypoglycemics (oral, eg, chlorpropamide)
•Hypnotics (eg, Phenobarbital)
•Quinidine
•Propylthiouracil
WHAT MAKES OUR TREATMENT DIFFERENT
- At World Stem Cell Clinic and The Royal British Medical Center we practice “Patient Precision Medicine (PPM)” which is a treatment model that proposes the customization of the treatment to each unique patient based on their medical history, stage of disease, exam results, time available for treatment and a patient orientation meeting with our Doctors before determining the best treatment for each unique patient.
- Our staff physicians are all board certified, in their field with years of experience. Your team includes both primary and ancillary care professionals devoted to maximizing your benefits from the procedures. We enroll you in an open registry to track your changes independently, for up to 5 years.
- To maintain our “Patient Precision Medicine (PPM)” services for you we may use peripheral blood, bone marrow, adipose or umbilical cord derived cells, plasma, proteins and Extracellular Vesicles based your unique treatment needs with mutual agreement.
- As our patient we also keep you abreast of the newest developments in treatment research. This is an ongoing relationship to maintain and enhance your health.
- Our promise is to provide you with travel and lodging support, access to bilingual staff members throughout the entire process and most importantly the best medical care possible.
