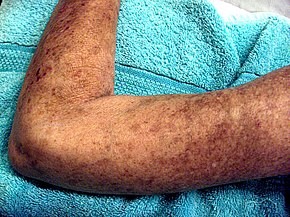
Scleroderma is a group of autoimmune diseases that may result in changes to the skin, blood vessels, muscles, and internal organs. The disease can be either localized to the skin or involve other organs as well. Symptoms may include areas of thickened skin, stiffness, feeling tired, and poor blood flow to the fingers or toes with cold exposure. One form of the condition, known as CREST syndrome, classically results in calcium deposits, Raynaud’s syndrome, esophageal problems, thickening of the skin of the fingers and toes, and areas of small dilated blood vessels.
The cause is unknown; however, some suspect it may be due to an abnormal immune response. Risk factors include family history, certain genetic factors, and exposure to silica. The underlying mechanism involves the abnormal growth of connective tissue which is believed to be the result of the immune system attacking healthy tissues. Diagnosis is based on symptoms, supported by a skin biopsy or blood tests.
While there is no cure, treatment may improve symptoms. Medications used include corticosteroids, methotrexate, and non-steroidal anti-inflammatory drugs (NSAIDs). Outcome depends on the extent of disease. Those with localized disease generally have a normal life expectancy. In those with systemic disease typical life expectancy is about 11 years from onset. Death is often due to lung, gastrointestinal, or heart complications.
About 3 out of 100,000 people per year develop the systemic form. The condition most often begins in middle age.[1] Women are more often affected than men. Scleroderma symptoms were first described in 1753 by Carlo Curzio and then well documented in 1842. The term is from the Greek skleros meaning “hard” and derma meaning “skin”.