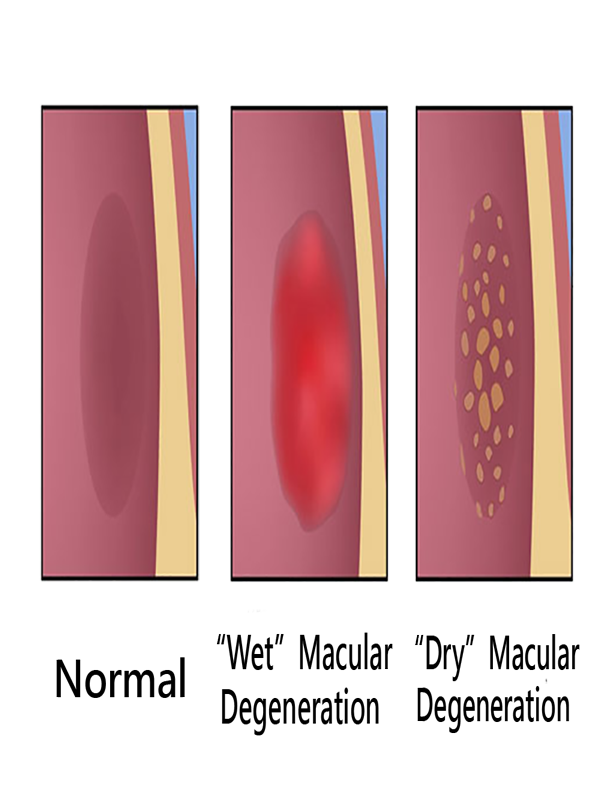
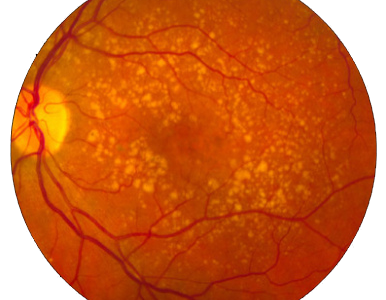
In the dry (nonexudative) form, cellular debris called drusen accumulate between the retina and the choroid, and the retina can become detached.
In the wet (exudative) form, which is more severe, blood vessels grow up from the choroid behind the retina, and the retina can also become detached.
It can be treated with laser coagulation, and with medication that stops and sometimes reverses the growth of blood vessels. The is a substantial amount of animal model studies showing that stem cells can reverse and or stabilize the vascularization processes, in the retina.
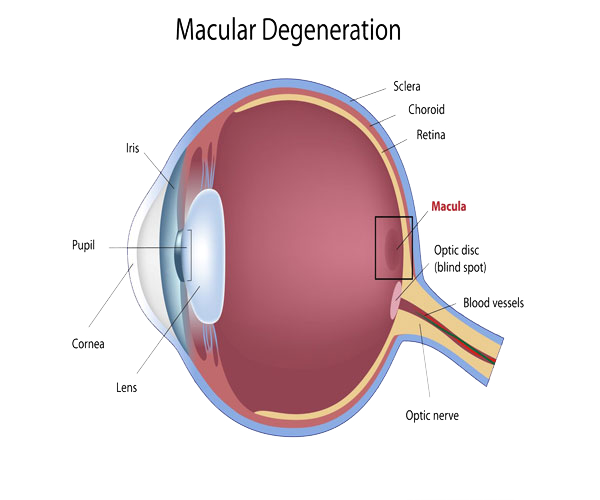
Although some macular dystrophies affecting younger individuals are sometimes referred to as macular degeneration, the term generally refers to age-related macular degeneration (AMD or ARMD).
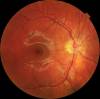
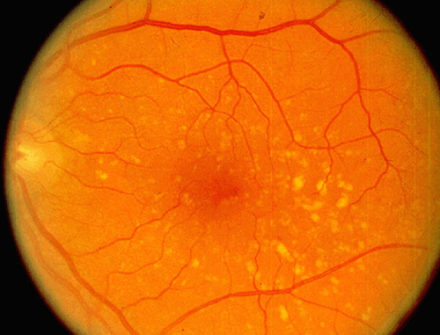
Age-related macular degeneration begins with characteristic yellow deposits (drusen) in the macula, between the retinal pigment epithelium and the underlying choroid. Most people with these early changes (referred to as age-related maculopathy) have good vision. People with drusen can go on to develop advanced AMD. The risk is considerably higher when the drusen are large and numerous and associated with disturbance in the pigmented cell layer under the macula. Recent research suggests that large and soft drusen are related to elevated cholesterol deposits and may respond to cholesterol-lowering agents.
Dry AMD:
Central geographic atrophy, the “dry” form of advanced AMD, results from atrophy to the retinal pigment epithelial layer below the retina, which causes vision loss through loss of photoreceptors (rods and cones) in the central part of the eye. No medical or surgical treatment is available for this condition, however vitamin supplements with high doses of antioxidants, lutein and zeaxanthin, have been suggested by the National Eye Institute and others to slow the progression of dry macular degeneration and, in some patients, improve visual acuity.